Blog Archives tasubtitleX
The RP-1 is a real fuel that is a complex hydrocarbon mixture (see above), therefore their thermodynamic properties considerably depend on composition. Surrogate mixtures represent complex aviation and rocket fuels such as RP-1 and RP-2 were proposed by Edwards and Maurice [11], Farmer et al. [12], Wang [13], and Huber et al. [14], [15].

What is Ideal Gas and Non Ideal Gas ideal gas vs real gas YouTube
Such data are presented here for the two rocket propellants RP-1 and RP-2. They were obtained using three different instruments to measure density, speed of sound, and viscosity at atmospheric pressure, and compressed liquid density in the combined range from 270 to 470 K with pressures to 40 MPa. The measurements reported here were part of a.

Captulo 7 Gases Ideales INDICE Introduccin Diagrama Pv
This means that we can also write the ideal gas equation as \ (PV=nRT=n\overline {N}kT\). Because the number of molecules in the sample, \ (N\), is \ (N=n\overline {N}\), we have. \ [PV=NkT. \nonumber \] This page titled 2.7: The Ideal Gas Constant and Boltzmann's Constant is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or.
Ecuación de los gases ideales (PV=nRT) YouTube
Turbulent two-phase reacting flow in the chamber of LOX/RP-1 bipropellant liquid rocket engine is numerically investigated in this paper. The predicted pressure and mean axial velocity are qualitatively consistent with the experimental measurements. The self-excited pressure oscillations are obtained without any disturbance introduced through the initial and boundary conditions. It is found.
Relacion Entre Las Capacidades Calorificas De Un Gas Varios Gas
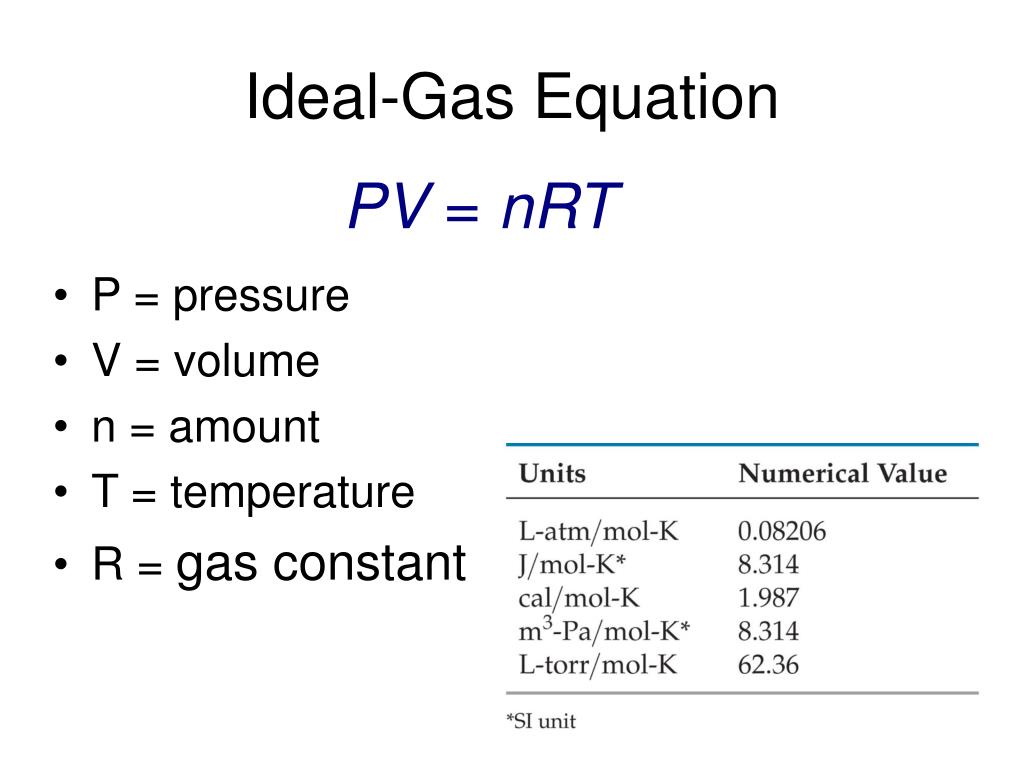
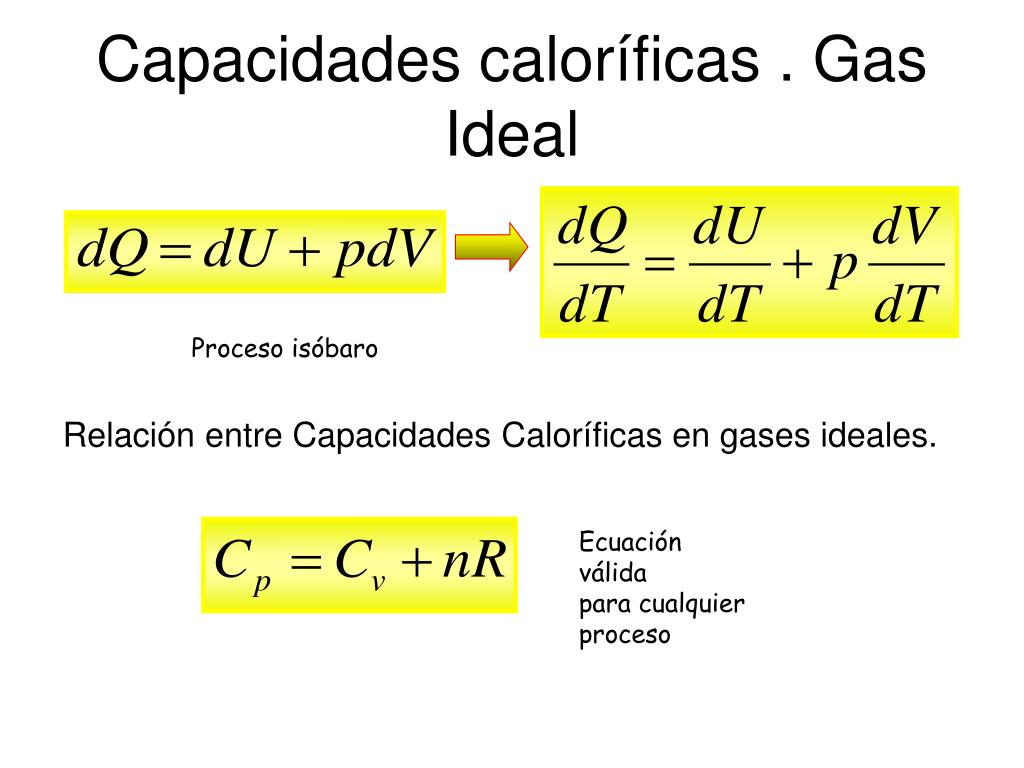
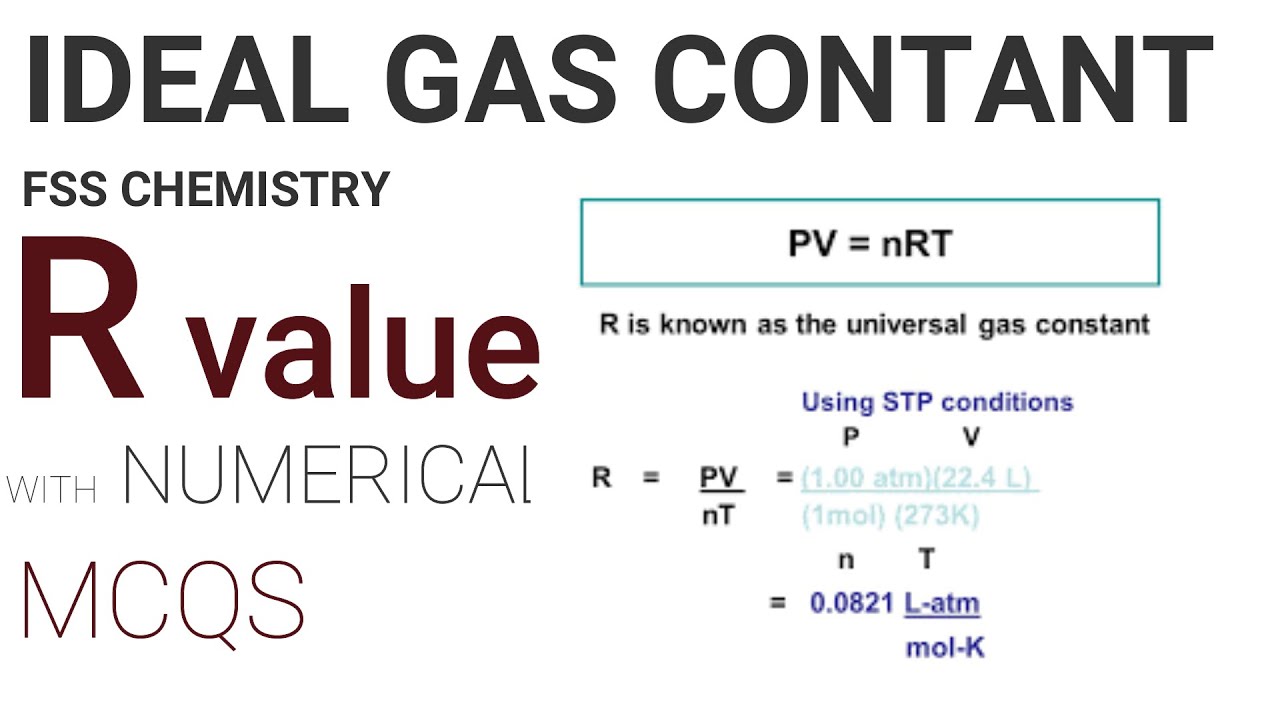
The gas constant (R) is a proportionality constant used in the ideal gas law and Nernst equation. It's also called the ideal gas constant, universal gas constant, or molar gas constant. Basically, the gas constant is the same as the Boltzmann constant (k), except the gas constant includes Avogadro's number (N A ): R = NA k.
[Solved] Ejemplo Un gas ideal es aquel para el cual PV/T es una
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol R or R.It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, rather than energy per temperature increment per particle.The constant is also a combination of the constants from Boyle's law.

Ecuación del gas ideal (Ejemplo paso a paso y valores de R) YouTube
RP-1 (alternatively, Rocket Propellant-1 or Refined Petroleum-1) is a highly refined form of kerosene outwardly similar to jet fuel, used as rocket fuel. RP-1 provides a lower specific impulse than liquid hydrogen (H 2 ), but is cheaper, is stable at room temperature, and presents a lower explosion hazard. RP-1 is far denser than H 2, giving it.

Ecuación del gas ideal PV=nRT YouTube
RP-1 is a long-established hydrocarbon fuel that continues to be widely used as the kerosene component in rocket propulsion systems. The desire in recent years to use rocket engines many times, rather than a single time, has led to reformulations of RP-1 and to the formulation of RP-2. In terms of processing, increased hydro-treating of the component feedstock fluids used in the manufacture of.

Temperatura de un Gas Ideal YouTube
of combustion, and aromatic content.1 Previous analysis of RP-1 has shown the fuel to be a complex mixture of compounds including paraffins, olefins, and aromatics.2 Although the sulfur concentration specification for RP-1 was set at 500 ppm (mass/ mass), the typical as-delivered lot was much lower at 30 ppm (mass/mass).

Ecuacion del gas ideal YouTube
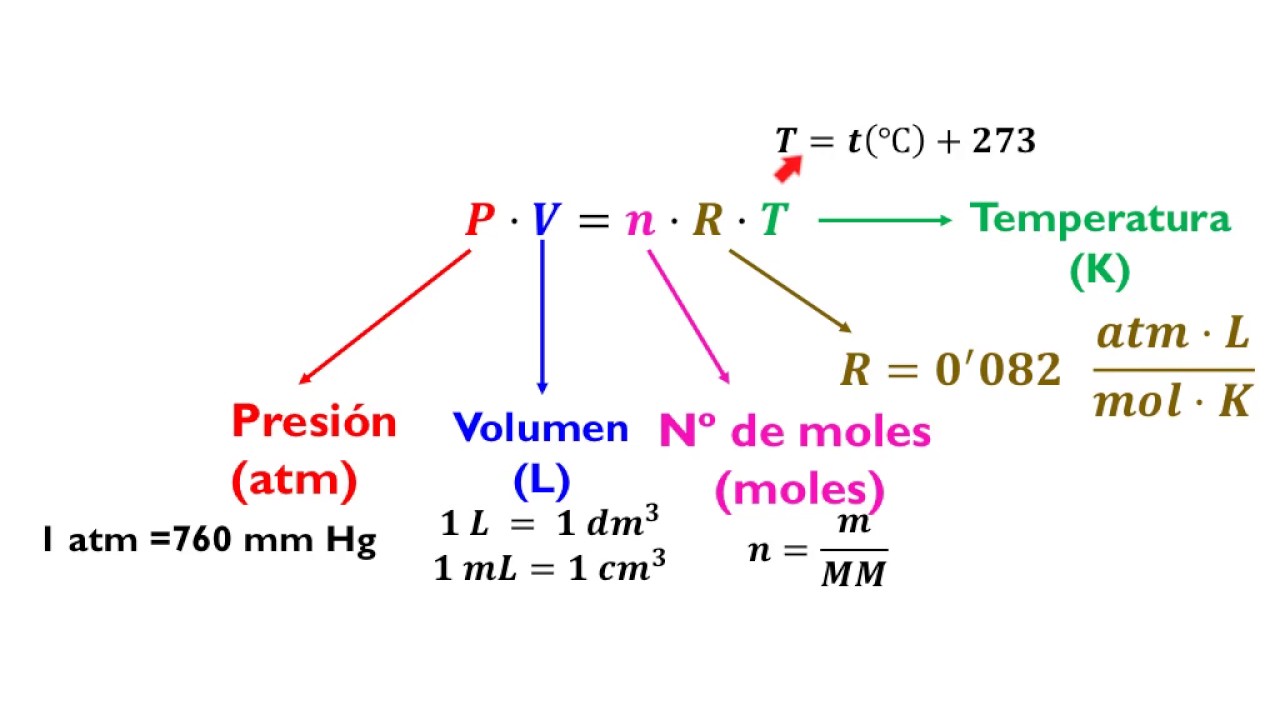
The ideal gas law has the form: PV = nRT, (12.4.14) (12.4.14) P V = n R T, where R is the universal gas constant, and with it we can find values of the pressure P, volume V, temperature T, or number of moles n under a certain ideal thermodynamic condition.

¿Qué es el Gas Ideal? YouTube
• Much more oxidizer than fuel (O/F >1) - Only LOX rich staged combustion systems are Russian engines - LOX/H2 systems typically utilize fuel rich pre burners 5 Karabeyoglu - Need a working gas to drive the turbine of the turbopump system - Vaporize the propellant to drive the turbine by • Combustion (very lean or

LEY DE LOS GASES IDEALES O ECUACIÓN DE ESTADO DEL GAS IDEAL YouTube
Contraction ratio of convergent segment 1.6 Mass flow rate of RP-1 (kg s 1) 3.4926 Nozzle expansion ratio 1.6 Mass flow rate of LOX (kg s 1 ) 8.0285 Throat size (cm) 34.125 Temperature of RP-1 (K) 485

The Boltzmann Equation Theory And Applications Download Free Read
Egolfopoulos and Law (1990) used a nozzle-burner counterflow method to make measurements from 9% (lean) to 35% (moderately rich) H2 molar concentration. This is a steady flow method which involves "the establishment of two symmetrical, planar, nearly-adiabatic flames in a nozzle- generated counterflow".

seco Contrato incrementar ecuacion de gases ideales Lingüística Glamour
La constante universal de los gases ideales, constante de los gases o constante molar de los gases 1 2 3 es una constante física, más concretamente termodinámica, que establecida inicialmente en relación con variables del estado gaseoso: volumen, presión, temperatura y cantidad de sustancia, ha devenido en una constante de gran importancia.
Ideal Gas Constant R EQUATION CHEMISTRY PV=nRT class 11 1st year
The proportionality constant, R, is called the gas constant and has the value 0.08206 (L•atm)/(K•mol), 8.3145 J/(K•mol), or 1.9872 cal/(K•mol), depending on the units used. The ideal gas law describes the behavior of an ideal gas , a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the.

Utilizando la ecuación de gas ideal. Calcule el valor de la constante
C12H234 were used to represent kerosene, whereas C12H268'9 was used to replace RP-1. The simplistic nature of these one-formula fuel models makes them easy to use. However, these simple formulas also present subtle problems when used in performance calculations. For example, when comparing to the physical-chemical properties of kerosene and RP.
- Fichas Del Abecedario Para Imprimir Pdf
- Como Hacer Puntos Basicos De Crochet
- T Shirt Basic Calvin Klein
- Biblia De La Conferencia Episcopal Española
- Planos De Edificios De 5 Pisos
- Macdonald Hill Valley Hotel Golf Spa
- Mercedes Viano 2 2 Cdi Opiniones
- Parte Europeo De Accidente Pdf
- Mejor Actuacion De La Historia Del Cine
- Troia Residence Apartamentos Marina S Hotels Collection Troia Carvalhal Grandola