
How to balance Ca(OH)2 + HCl = CaCl2 + H2O YouTube
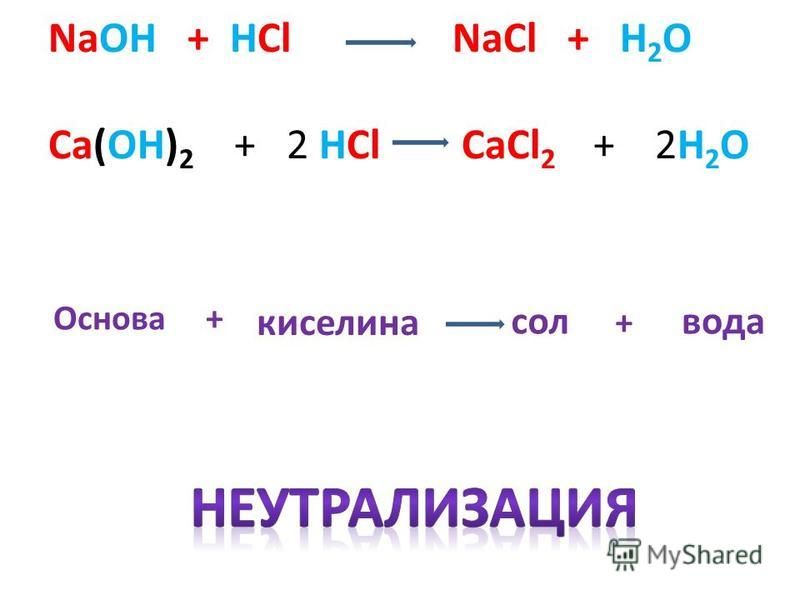
The balanced chemical equation is Ca(OH)₂ + 2HCl ⇒ CaCl₂ + 2H₂O. The balancing of the chemical equation is done by balancing all the atoms on the RHS of the equation and on the LHS of the equation.; After balancing the sum of the atoms on the RHS must be equal to sum of the atoms on LHS.; The balancing of the chemical equation is done by adding a suitable coefficient before the atoms.

Equation for Calcium Hydroxide Dissolving in Water Ca(OH)2 + H2O YouTube
CaCl 2. Name: calcium chloride . Atomic weight: 110.9840 . Boiling point: 1600°C . Melting point: 772°C
Type of Reaction for HCl + Ca(OH)2 = CaCl2 + H2O YouTube
Step 1/2. First, we need to make sure that the number of atoms of each element is the same on both sides of the equation. Ca (OH)2 + 2HCl → CaCl2 + 2H2O Now, we have two atoms of hydrogen and two atoms of chlorine on both sides. We also have one atom of calcium and two atoms of oxygen on the left side, and one atom of calcium and two atoms of.

2 Phase isopleth of (Ca(OH)2 CaCl2 H2O) ternary system (Qiao et al.,... Download Scientific
Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. Example: H 2 + O 2 = H 2 O. Count the number of H and O atoms on both sides. There are 2 H atoms on the left and 2 H atom on the right.

CaO+HCl=CaCl2+H2O Balanced EquationCalcium oxide+Hydrochloric acid=Calcium chloride+Water
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. CaCl2 + 2 H2O = Ca (OH)2 + 2 HCl. Reactants.

When NH4Cl is treated with CaO we get CaCl2, Ca(OH)2 and X as its products. Where X is
There are three main steps for writing the net ionic equation for HCl + Ca(OH)2 = CaCl2 + H2O (Hydrochloric acid + Calcium hydroxide). First, we balance the.

How to Write the Net Ionic Equation for CaCl2 + KOH = Ca(OH)2 + KCl YouTube
HCl + Ca(OH)2 = CaCl2 + H2O - Ecuación química balanceada. Ecuación química balanceada. 2 HCl + Ca(OH) 2 → CaCl 2 + 2 H 2 O . reacción química . Cloruro De Hidrógeno + Hidróxido De Calcio = Cloruro De Calcio + Agua. estructura química. Tipo de reacción. Doble desplazamiento (Ácido-Base) Lista de Ácidos HCl

exercises Zn + HCl → ZnCl2 + H2 HCl + Ca(OH)2 → CaCl2 + H2O Al2O3 + H2SO4 → Al2(SO4)3 +H2O P
In order to balance O on both sides we: Multiply coefficient for H 2 O by 2. 2 HCl (aq) + 1 Ca (OH) 2 = 1 CaCl 2 + 2 H 2 O. H is balanced: 4 atoms in reagents and 4 atoms in products. All atoms are now balanced and the whole equation is fully balanced: 2 HCl (aq) + Ca (OH) 2 = CaCl 2 + 2 H 2 O.

Ca(OH)2 + HCl > CaCl2 + H2O Brainly.in
Word Equation. Hydrogen Chloride + Calcium Hydroxide = Calcium Chloride + Water. HCl + Ca(OH)2 = CaCl2 + H2O is a Double Displacement (Acid-Base) reaction where two moles of aqueous Hydrogen Chloride [HCl] and one mole of solid Calcium Hydroxide [Ca(OH) 2] react to form one mole of aqueous Calcium Chloride [CaCl 2] and two moles of liquid Water [H 2 O]
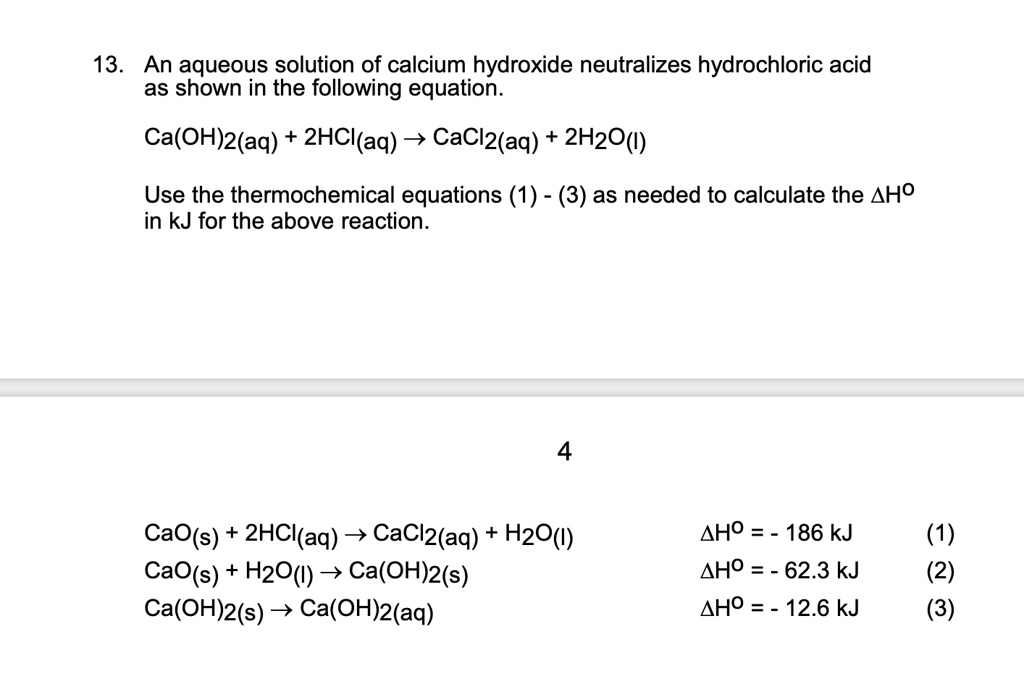
SOLVED 13 An aqueous solution of calcium hydroxide neutralizes hydrochloric acid as shown in
Balance the following equation:Ca(OH)2 + HCl → CaCl2 + H2OSUBSCRIBE if you'd like to help us out!https://www.youtube.com/channel/UC2C34WdYMOm47PkWovvzLpw?sub.

CaCl2Ca(OH)2H2O phase diagram for different Ca(OH)2/CaCl2 molar... Download Scientific Diagram
Yes, this is a balanced equation because there are the same amounts of Calcium and clorine atoms either side of the equation. Study with Quizlet and memorize flashcards containing terms like Balance the chemical equation Ca (OH)2+HCL > CaCl2 + H20, Balance the chemical equation Na+I2> NaI, State the word equation for sodium and chlorine.

balance the equation by full method÷Ca(OH)2+HCl}CaCl2+H2O Brainly.in
Reaction of Ca(OH) 2 and HCl and balanced equation As mentioned earlier, calcium chloride and water are given as products. Because Calcium hydroxide's solubility is low in water, Ca(OH) 2 partially dissociates to Ca 2+ and OH-ions. Therefore there is very low OH-concentration in the aqueous solution which contains Ca(OH) 2.; But, HCl is readily soluble in water dissociates to H + ions and Cl.

how to balance caco3 hcl cacl2 co2 h2o YouTube
Word Equation. Calcium Hydroxide + Hydrogen Chloride = Calcium Chloride + Water. Ca(OH)2 + HCl = CaCl2 + H2O is a Double Displacement (Acid-Base) reaction where one mole of solid Calcium Hydroxide [Ca(OH) 2] and two moles of aqueous Hydrogen Chloride [HCl] react to form one mole of aqueous Calcium Chloride [CaCl 2] and two moles of liquid Water [H 2 O]

Balancea y comprueba las siguientes ecuaciones químicas por el método de tanteo1. Ca + HCl →
¡Balancea la ecuación o reacción química Ca(OH)2 + HCl = CaCl2 + H2O utilizando la calculadora!
Hcl Ca Oh 2 Cacl H2o EDUCA
In this video we'll balance the equation HCl + Ca(OH)2 = CaCl2 + H2O and provide the correct coefficients for each compound. To balance HCl + Ca(OH)2 = CaCl.

Describe Qué tipo de reacción es HCI + Ca(OH)2 CaCl2 + H20 ? Brainly.lat
VIDEO ANSWER: people. Sorry. It's with the balance of the Fio two. You owe us this guy calcium. Fine. Oh, three plus water yields silicon dioxide wass calcium.
- 2019 Hyundai Ioniq Hybrid Interior
- Agregar Seccion Desactivado Powerpoint 10
- Una Casa Que Sea Bonita Y Una Piscina Para Nadar
- Festival De Jazz De Tanger
- Velocidad Sf Monsieur Cuisine Connect
- Apple Lightning Av Adapter Netflix
- Ak 550 Que Hace El Panel Central
- Is The Gaming Industry Dying
- Chocolate Blanco En Polvo
- Busca La Definición De Tasa De Error En Una Transmisión